Nature talks to vaccine developer Kayhan Azadmanesh about efforts in Iran to develop ten or more COVID jabs, two of which have been approved for use.
Iran was among the first countries to be hit with an outbreak of COVID-19 in early 2020. It is currently battling its fifth wave, likely driven by the Delta variant. Official figures suggest that more than 4.3 million people have been infected and 97,000 have died since the pandemic began, but the true toll is potentially much higher.
Scientists say Iran is one of few Middle Eastern nations with the capacity to develop vaccines. It has been doing so in earnest: around ten are under development and one is already bolstering its vaccination drive, but little is known about these vaccines outside Iran.
Nature speaks to Kayhan Azadmanesh, a medical doctor and biotechnologist who is head of the virology research division at the Pasteur Institute of Iran in Tehran, about the nation’s vaccine landscape. Azadmanesh also advises the Iranian government and is developing two viral-vector vaccines through his spin-off company Humimmune Biotech.
How badly has the pandemic affected Iran?
Since January 2020, we’ve had five separates waves. We’re currently experiencing the highest number of new cases reported so far, with around 40,000 a day, and the most common variant we detect is Delta. But many more cases are likely going unreported. The outbreak is putting pressure on hospitals and the situation is not looking good.
Which COVID-19 vaccines are available in Iran?
So far, 18 million or so doses have been administered: some 12 million were China’s Sinopharm vaccine; 4 million were the Oxford–AstraZeneca vaccine; and 1 million were COVIran Barekat, developed by the Iranian state-owned Shifa Pharmed Industrial Group in Tehran. The remainder include doses of Russia’s Sputnik V and India’s Covaxin. More than half a million doses are being administered a day, and some 17% of Iran’s population of 85 million have received their first dose of a COVID-19 vaccine.
Could you tell us about COVIran Barekat?
It is an inactivated vaccine and is still undergoing phase III trials, but it received emergency-use authorization in June. It was approved on the basis of the levels of antibodies it induces, including those that can ‘neutralize’ SARS-CoV-2, or block it from entering cells. In early trials, the researchers found that more than 93% of vaccinated people produced neutralizing antibodies. We don’t know how long this protection will last, but I assume that it will be similar to that provided by other inactivated vaccines — such as CoronaVac, produced by the Chinese firm Sinovac Life Sciences — for which antibody levels have been shown to drop after six months1, suggesting that boosters are likely to be required.

Kayhan Azadmanesh holding rapid-antibody tests that show a negative result before, and a positive result after, he received a COVID-19 vaccination.
What other vaccines are being developed in Iran?
Pasteurcovac is a recombinant-protein vaccine developed in a collaboration between Cuba’s Finlay Institute of Vaccines in Havana and the Pasteur Institute of Iran. The vaccine is known as Soberana 02 in Cuba. It also received emergency-use approval in Iran in June, despite still being in phase III trials. There are several other inactivated vaccines and recombinant-protein vaccines in clinical trials, and there is at least one mRNA vaccine, two adenovirus-vector vaccines and one measles-virus-vector vaccine in earlier stages of development. Vaccines developed outside Iran are also currently in clinical trials and being produced locally.
Tell me about the vaccines you are designing?
My company, Humimmune Biotech, has been working on two vaccine candidates. One uses the measles virus as a backbone to introduce a gene that encodes either the SARS-CoV-2 spike protein, which the virus uses to enter cells, or the nucleocapsid protein that it requires to replicate. That vaccine is being produced by the Iranian firm BioSun Pharmed in Tehran.
The other vaccine, which might be more promising, uses an adenovirus 5 backbone to deliver part of the sequence for the spike protein — a similar backbone to that used in the second dose of Sputnik V. We hope to start clinical trials early next year. Most of the COVID-19 vaccines used in Iran so far have been inactivated vaccines, which I expect will mean people will need booster shots next year. Our vaccine could be used as a booster, and a mix-and-match approach might even offer better protection. The technology can also be easily modified against new variants — we have already begun developing a version for Delta.
Why are Iranian scientists creating so many vaccines?
We have a long history of vaccine production in Iran. The Pasteur Institute of Iran was established in 1920, and has produced vaccines against tuberculosis and rabies. Vaccines have also been developed in Iran against measles, mumps and human papilloma virus.
We can’t rely on help from the international community with the pandemic. We are living under sanctions imposed by the United States; in our opinion, these are unjustified. The United States says that sanctions don’t affect humanitarian activities, but when your ability to transfer money is restricted, it is difficult to buy drugs and medicines. And we have the technology to produce vaccines, so why not use it? To ensure the safety of Iranians, it makes sense to develop a variety of vaccines using different research and development strategies, as China has done.
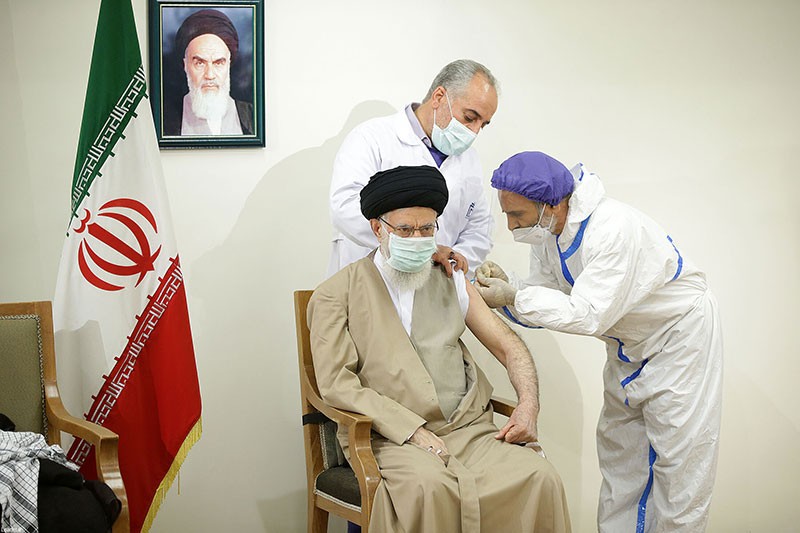
Iran's Supreme Leader Ayatollah Ali Khamenei receives a dose of the locally-made COVIran Barekat vaccine in June.
Why are Iranian researchers reluctant to publicize their work internationally?
This could be another side effect of the sanctions. Researchers in Iran might not want to draw too much attention to their work in case they put potential partnerships in jeopardy before they have achieved a final product, or they run the risk of losing access to raw materials and technologies they need for vaccines.
Researchers are also extremely busy, helping in the effort to fight the pandemic in Iran. They might not have time to publish results in international journals. But some have started to share results. In June, the researchers developing COVIran Barekat published a preprint of their preclinical results2, and they will share clinical results very soon. We also plan to share the results of our adenovirus-vector vaccine soon.
This could be another side effect of the sanctions. Researchers in Iran might not want to draw too much attention to their work in case they put potential partnerships in jeopardy before they have achieved a final product, or they run the risk of losing access to raw materials and technologies they need for vaccines.
Researchers are also extremely busy, helping in the effort to fight the pandemic in Iran. They might not have time to publish results in international journals. But some have started to share results. In June, the researchers developing COVIran Barekat published a preprint of their preclinical results2, and they will share clinical results very soon. We also plan to share the results of our adenovirus-vector vaccine soon.
What have been the biggest challenges in developing COVID-19 vaccines?
The sanctions have caused a lot of difficulty, because they make it hard for us to buy materials and equipment. For example, chromatography resins we need to purify vaccines are mostly produced by multinational companies that are major suppliers to the United States, so they might be afraid of selling to us. The United States says that we can apply for exemptions, but, in our experience, that hasn’t worked. But somehow, we find a way. We modify our methods, find other providers, or look for local solutions. We search for the best we can get, but sometimes quality and efficiency are affected.
Also, one of the biggest challenges globally is scale. Prior to the pandemic, Iran primarily had to produce vaccines for children, with a production requirement for each vaccine of around 3 million doses a year. Now we need about 170 million doses to fully vaccinate the whole population.
What does the future hold for vaccine development in Iran?
The initial target for COVIran Barekat was to produce up to 30 million doses a month by September, which would have been enough to vaccinate the adult population. But they have not been able to achieve that, so we have had to import millions of doses of other vaccines. As many people have said, this will not be the last coronavirus pandemic that we face. I expect the vaccine production capacity will be used for years to come to develop new vaccines and drugs, for both coronaviruses and other diseases.
doi: https://doi.org/10.1038/d41586-021-02216-z
No comments:
Post a Comment